Allogeneic hematopoietic cell transplantation (HSCT) has curative potential for Acute myeloid leukemia (AML). Given that the median age of acute myeloid leukemia (AML) patient is 68 years, the majority of HSCT candidates in AML are inevitably elderly patients. Advances in alternative donor HSCT and reduced intensity conditioning have expanded the opportunities for elderly patients to undergo HSCT. Previously, we reported HSCT outcomes of older adults with AML according to donor type, showing comparable outcomes among haploidentical donor (HID), matched unrelated donor (MUD) and matched sibling donor group, with especially superior survival in the MUD group ( Transplantation and Cellular Therapy 2021; 27: 774.e1-774.e12.). Since donor age has been shown to be a significant factor in HSCT outcomes, we investigated the HSCT outcomes of older AML HSCT recipients from HID according to donor age, and compared to those of MUD HSCT recipients.
We retrospectively evaluated older adults with AML aged 60 and above, who underwent first HSCT at Catholic Hematology Hospital between 2007 and 2022, in complete remission (CR) or CR with incomplete hematologic recovery. We analyzed the HSCT outcomes (Overall Survival [OS], Relapse-Free Survival [RFS], and Graft-versus-Host Disease [GVHD], Relapse-Free Survival [GRFS]) in patients who underwent either HID or MUD HSCT.
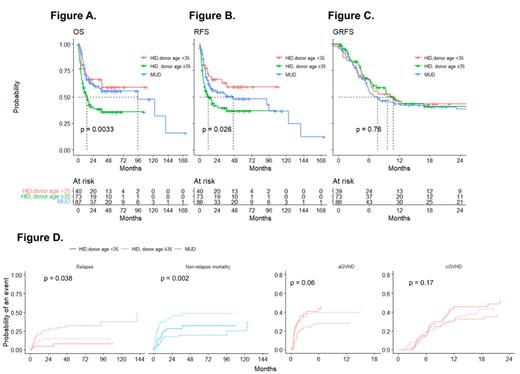
A total of 204 patients were included, with 117 (57.4%) in the HID group and 87 (42.6%) in the MUD group. For MUD HSCT, conditioning regimens consisted of cyclophosphamide 120 mg/kg combined with 1320 cGy of fractionated total body irradiation (TBI) or busulfex [12.8 mg/kg], or busulfex [6.4 mg/kg] and fludarabine [150 mg/m2] with 400 cGy of fractionated TBI. Conditioning for HID HSCT comprised of busulfex [6.4 mg/kg] and fludarabine [150 mg/m2] with 400-800 cGy of fractionated TBI. Rabbit antithymocyte globulin was administered at a dose of 1.25 mg/kg/day on days -3 to -2 for MUD HSCT and days -4 to -1 for HID HSCT. There was no significant difference in the clinical characteristics between the MUD and the HID groups, except donor age (MUD vs. HID: median 28 years vs. 37 years, p <0.01).
In the survival analysis, 196 patients whose donor age was identified were included. The difference in survival based on donor age was striking. Patients who received transplantation from donors aged below 35 showed superior outcomes compared to those with donors aged 35 and above (Figure A). The impact of donor age on survival differences was also pronounced in RFS (Figure B). However, the differences based on donor age were mitigated in terms of GRFS (Figure C), suggesting that donor age may play a role in the occurrence of GVHD and relapse.
In the cumulative incidence analysis, there was a significant difference in relapse and non-relapse mortality between patients who received HID HSCT from younger donors aged below 35, patients who received HID HSCT from older donors aged 35 and above, and patients who received MUD HSCT. The patients who underwent MUD HSCT showed a significantly higher relapse rate than those who underwent HID HSCT regardless of donor age. HID HSCT patients with older donors (aged 35 and above) showed significantly higher non-relapse mortality than the other two groups. In the analysis of cumulative incidence of GVHD, with death as a competing risk, patients who underwent MUD HSCT showed lower incidence of acute GVHD than those who underwent HID HSCT (Figure D).
Our data showed equally promising outcomes of HID HSCT from a younger donor (age <35) compared to MUD-HSCT, suggesting the necessity for more sophisticated strategies to improve survival outcomes in HID HSCT from older donors. Further studies comparing donor age are warranted in various transplantation settings.
Disclosures
Lee:Kira: Consultancy; Achillion: Research Funding; Alexion, AstraZeneca Rare Disease: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; AlloVir: Consultancy; Samsung: Consultancy; Arrowhead: Consultancy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal